How to pursue the deficiencies of mRNA vaccine of BioNTech from the both aspects of dsRNA and residual DNA
Focus on the "trade-off" between dsRNA and residual DNA.
I previously made the following post about dsRNA (ref. 1). This time, I will provide a more logical and concise explanation for the dsRNA and the residual DNA. All we need to do this time is to focus on the "trade-off" between the dsRNA and the residual DNA. The "trade-off" is an incompatible relationship in which one gains something and loses something else.
The dsRNA contamination and its risks in the mRNA vaccine, From BioNTech Patent.
I newly discovered BioNTech patent regarding a relationship between GTP/UTP concentrations and dsRNA, and hence I would like to report this here. I previously reported BioNTech patent US20230183769A1 regarding the ATP and CTP concentrations in connection with the Process 2 (ref. 1).
From the above post, we can understand that BioNTech seems to have wanted to reduce the dsRNA contamination in the mRNA vaccine.
The dsRNA contaminating the mRNA vaccine is to be recognized as a virus in the body and may cause inflammatory effects (side effects) due to cytokines, etc (ref. 2: Katalin Karikó, et al).
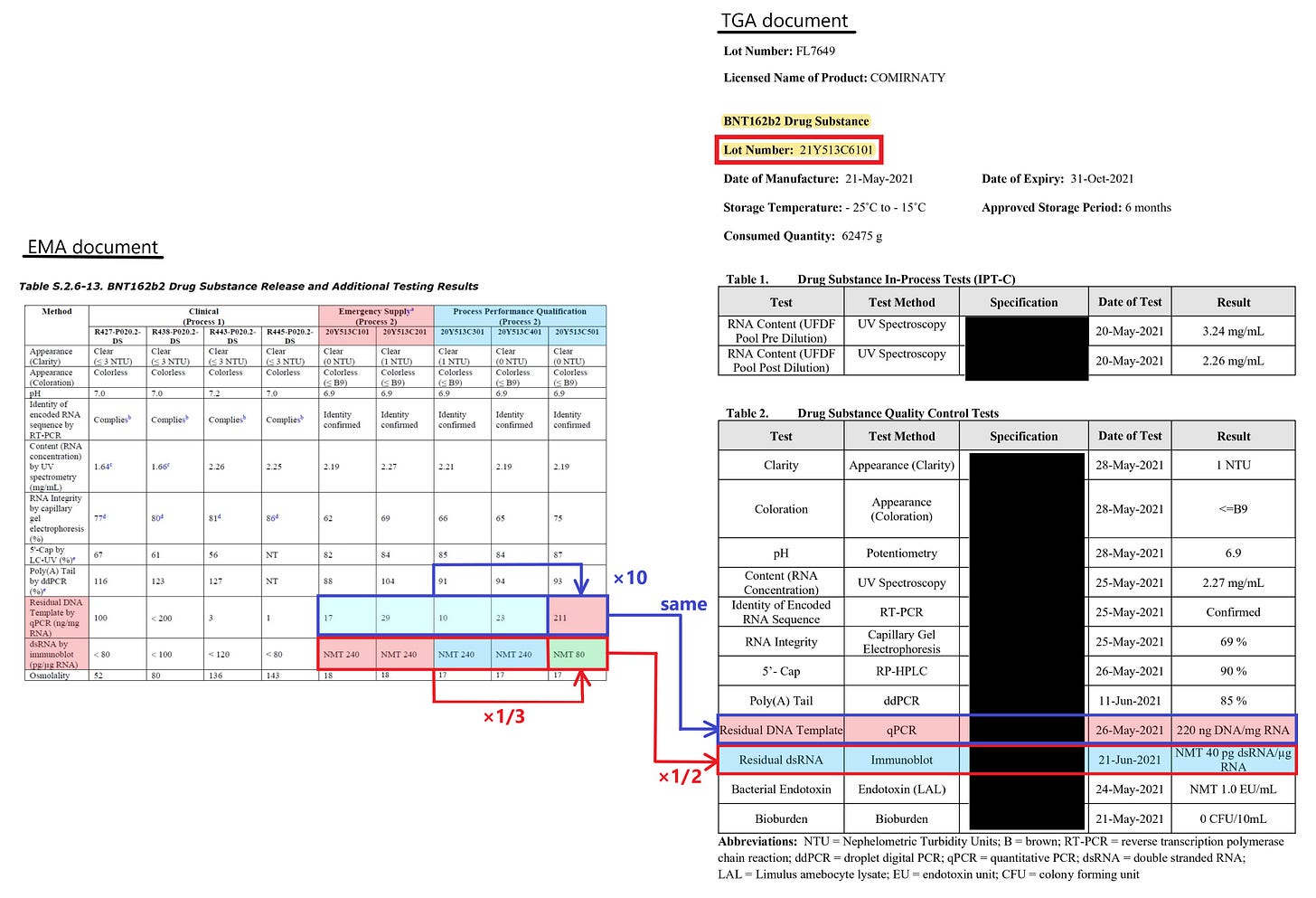
According to the EMA document (ref. 3), in the early stage of the introduction of mRNA vaccine (for example, during emergency supply), BioNTech supplied the vaccine with a relatively high dsRNA contamination level of not more than 240 pg/ug RNA (see Lot No. 20Y513C101, C201, C301, C401).
Later, BioNTech changed the manufacturing method to reduce the dsRNA contamination level to not more than 80 pg/ug RNA (see Lot No. 20Y513C501).
However, this change caused the residual DNA contamination level to increase by more than 10-fold. The amount of the residual DNA became 211 ng DNA/mg RNA from ten-digit levels.
The mechanism behind this phenomenon has been already described in the BioNTech patent US20230183769A1 (ref. 4, ref. 5).
As a matter of course, the amount of residual DNA contamination is a false value underestimated with the qPCR (ref. 6: Moderna patent WO2014152030A1, Stephane Bancel, et al).
In short, in the early stage of vaccine introduction, a vaccine with relatively high dsRNA contamination and relatively low residual DNA contamination was introduced into the body.
Then, in the subsequent design change, a vaccine with relatively low dsRNA contamination and relatively high residual DNA contamination was introduced into the body.
It is extreamly unnatural to increase the residual DNA contamination level by more than 10 times in order to reduce the dsRNA contamination level to 1/3.
The ideal solution is to reduce both.
Furthermore, BioNTech appears to have improved the manufacturing method thereafter, and the amount of dsRNA contamination in a subsequent Lot listed in the TGA document (ref. 7) has been reduced to not more than 40 pg/ug RNA (see Lot No. 21Y513C6101). However, the amount of the residual DNA is 220 ng DNA/mg RNA, and there is no improvement in the 10-fold residual DNA contamination.
The questions here are...
(1) Could this dsRNA contamination be related to the high number of serious side effects in the early stage of the introduction of mRNA vaccine?
(2) Could the variation in side effects between lots be due in part to the adjustment of the amount of dsRNA contamination?
240 pg/ug RNA or less → severe side effects ?
80 pg/ug RNA or less → mild side effects ?
40 pg/ug RNA or less → milder side effects ?
(3) The amount of residual DNA has been increased by changing the manufacturing method and underestimated by the qPCR, but is this truely safe and effective?
(4) Could the dsRNA be involved in the early side effects, and could the residual DNA be involved in the late side effects?
(5) BioNTech developed the vaccine at the speed of science, so perhaps did they fail to adjust the dsRNA and residual DNA contaminants?
There is no information or research in the EMA document to suggest safety from the dsRNA contamination, and there appears to be no threshold for dsRNA contamination to ensure safety. The same is true for the DNA contamination.
The side effects caused by the dsRNA and the residual DNA need to be observed separately, but it seems to be enough for pharmaceutical companies to hide the severe early side effects.
Maria Gutschi san made a good point in response to my post.
For this point, I have a same opinion. In regard to Moderna, we need to pay attention to the larger vaccination volume and unknown amount of the dsRNA contamination.
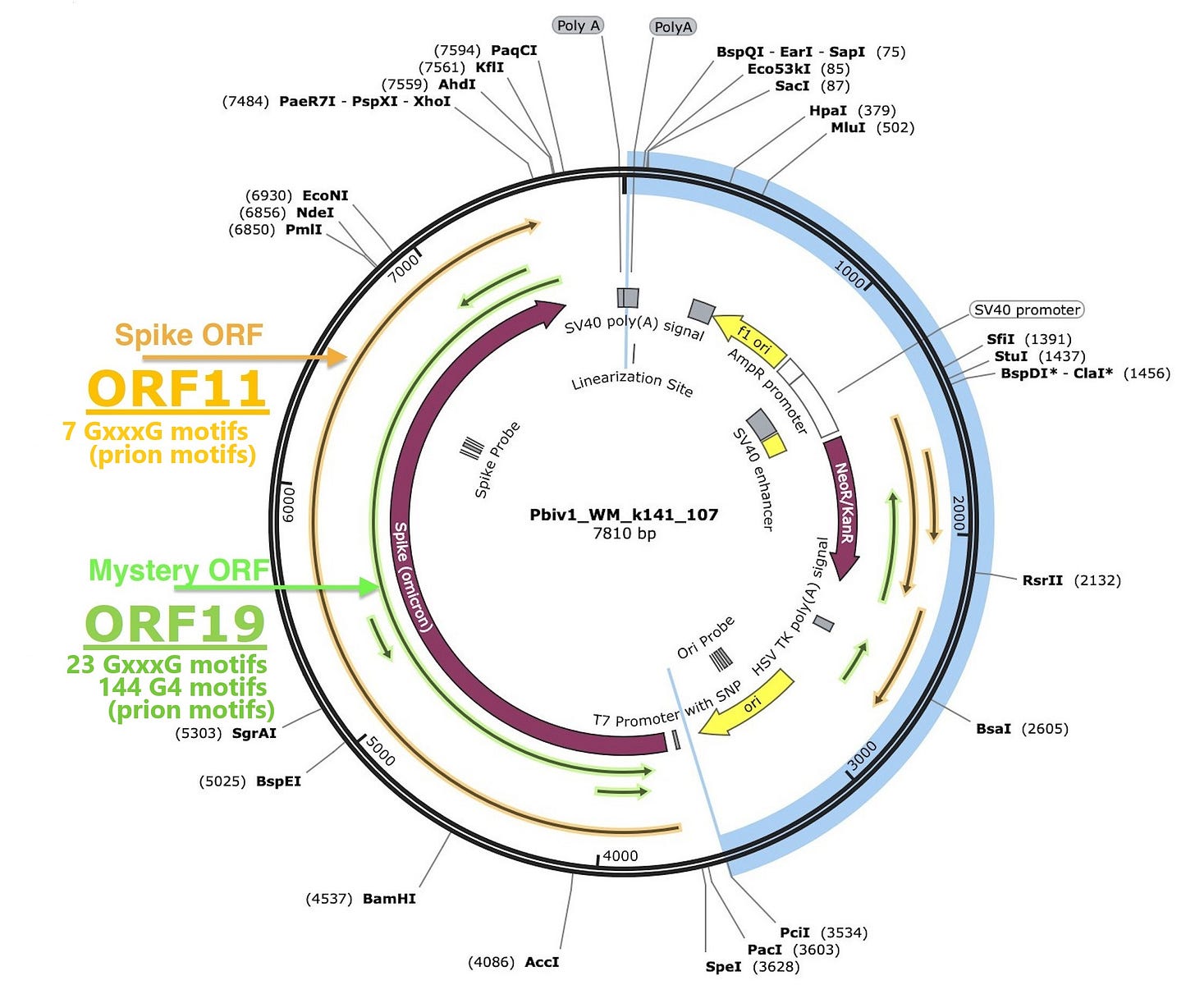
On the other hand, in regard to BioNTech, since the dsRNA may be encoding the antisense (cDNA) of the spike, we may also need to pay attention to the antisense as with the spike. The antisense of the spike may have a prion motif-rich structure (see green mystery ORF).
McKernan san made further deep consideration to the dsRNA.
Reference list:






Excellent analysis.
I have been thinking there has been a trade off for about a year now, but your discovery of the patent and the publishing of it by BioNTech scientists confirmed it for me. Although many say the LNPs or endotoxin are the adjuvants, I believe it is either dsRNA or rDNA that are acting as the adjuvants.
So the manufacturers NEED to have it a little bit "dirty" in order to get an immune response.
Pardi says it himself here: https://www.ema.europa.eu/en/documents/presentation/presentation-innate-immune-mechanisms-mrna-vaccines-rein-verbeke-et-al_en.pdf
I write about it in my very first substack though I didn't know about the IVT method used by BioNTech.
https://mariagutschi.substack.com/p/a-washed-up-pharmacists-musings
See also the brilliant work by Ganna Petruk and her team showing the intimate binding of mRNA, dsDNA to Endotoxin and the impact on casualty rates. They deserve a Nobel Prize.
https://portal.research.lu.se/en/persons/ganna-petruk
discussed in
https://geoffpain.substack.com/p/gmo-spike-protein-carries-e-coli
and
https://geoffpain.substack.com/p/covid19-deaths-and-ongoing-symptoms
and
https://geoffpain.substack.com/p/late-migrating-species-in-pfizer