New Drug Delivery Systems: From BioNTech patent
BioNTech proposes complexes containing cationic and/or ionizable oligosaccharides.
A recently published BioNTech patent US20250057960A1 (Filing date: Oct, 21, 2022) proposes complexes containing cationic and/or ionizable oligosaccharides to address the safety concerns (specifically, the lack of satisfactory safety and efficacy) associated with lipid-based drug delivery systems (DDS), including lipid nanoparticles (LNPs).
They must be aware of the specific adverse events related to LNPs. If LNPs were safe and effective, there would be no need to develop new technologies.
I believe that BioNTech's stance of continuing to use LNP as a drug delivery system for mRNA while being aware of the safety concerns surrounding LNP is unethical. In the future, will contaminants such as residual DNA, dsRNA, endotoxins, etc. be delivered by oligosaccharide complexes?
https://patents.google.com/patent/US20250057960A1/en?oq=US20250057960
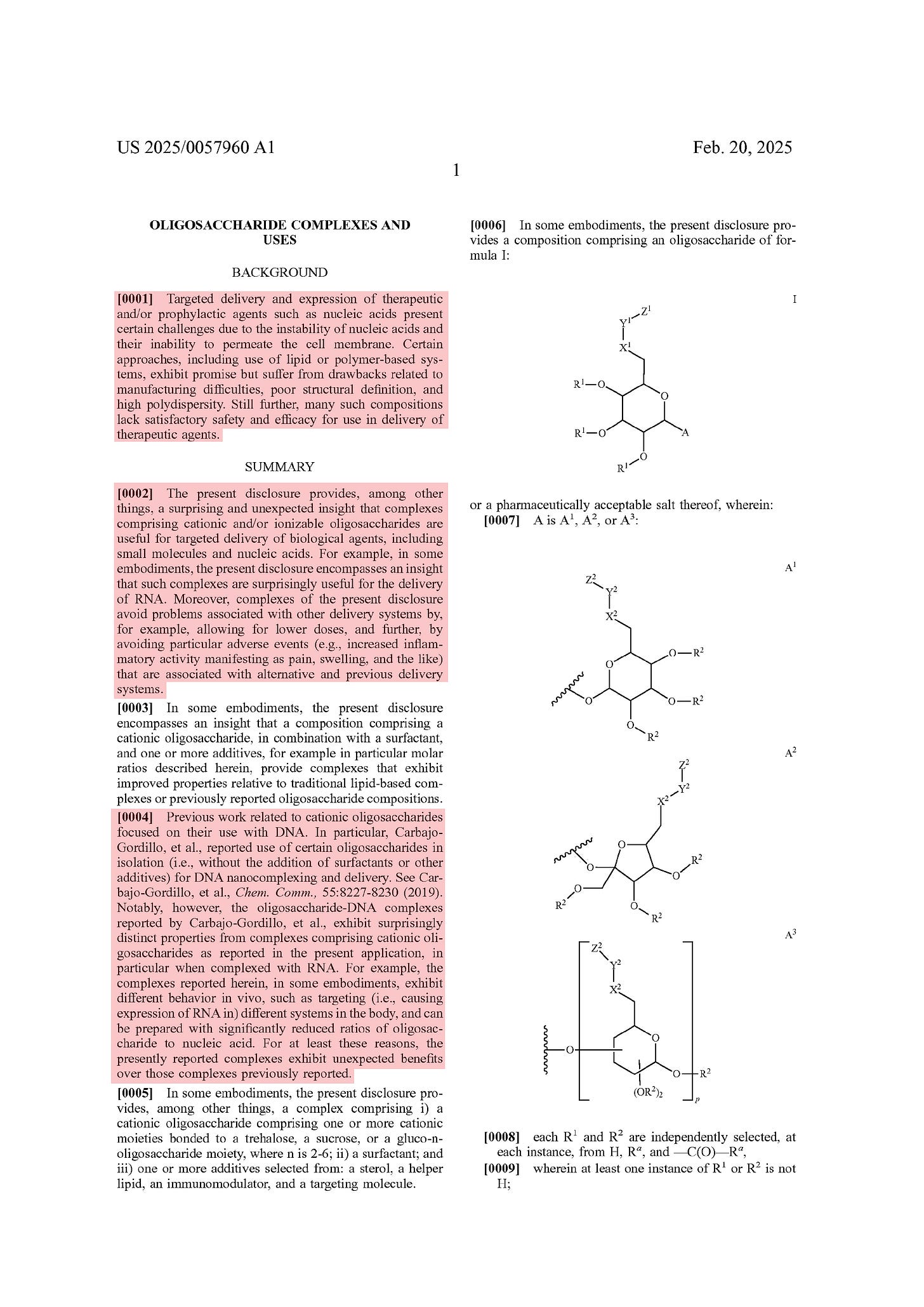
BACKGROUND
[0001] Targeted delivery and expression of therapeutic and/or prophylactic agents such as nucleic acids present certain challenges due to the instability of nucleic acids and their inability to permeate the cell membrane. Certain approaches, including use of lipid or polymer-based systems, exhibit promise but suffer from drawbacks related to manufacturing difficulties, poor structural definition, and high polydispersity. Still further, many such compositions lack satisfactory safety and efficacy for use in delivery of therapeutic agents.
SUMMARY
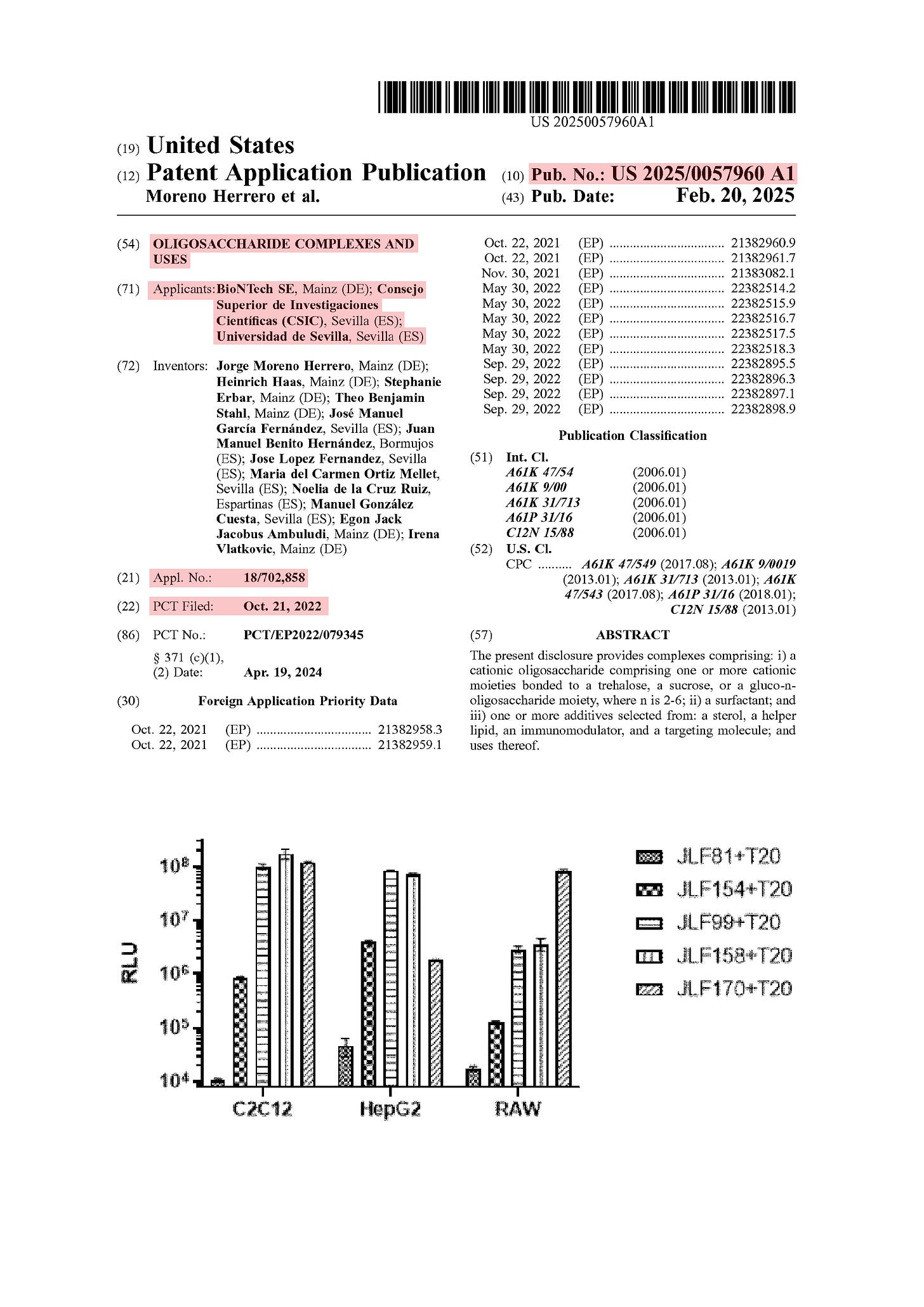
[0002] The present disclosure provides, among other things, a surprising and unexpected insight that complexes comprising cationic and/or ionizable oligosaccharides are useful for targeted delivery of biological agents, including small molecules and nucleic acids. For example, in some embodiments, the present disclosure encompasses an insight that such complexes are surprisingly useful for the delivery of RNA. Moreover, complexes of the present disclosure avoid problems associated with other delivery systems by, for example, allowing for lower doses, and further, by avoiding particular adverse events (e.g., increased inflammatory activity manifesting as pain, swelling, and the like) that are associated with alternative and previous delivery systems.


Inventors, Test yourself for 3 years before doing it !!
Look at the stupidity of Japanese public health authorities.
Vaccination rate at the time of each dose completion
First time February 17, 2021 80.8%
Second time in June 2021? 79.9%
Third time December 1, 2021 67.5%
4th May 25, 2022 45.6%
5th August 1st, 2022 27.2%
6th May 8, 2023 17.5%
7th September 20, 2023 13.9%
8th October 2024 - 10% (estimated from Nagoya-City)
the result
All causes of deaths (population abt. 124 million)
2017 1340567
2018 1362470
2019 1381093 100% (based on this year)
2020 1372755 99.4%
2021 1439856 104.2% (BNT162b2 starts in February)
2022 1569050 113.6%
2023 1575936 114.1%
2024 1618684 117.2% (breaking news)
https://www.e-stat.go.jp/stat-search/file-download?statInfId=000040248806&fileKind=2
Even if the average vaccination rate is simply 42.8%, vaccination death is ≒ 1.27%
Side effects of conventional vaccines: mortality rate <1 in 1 million people (0.0001%)
I learnt from Geoff Pain that the Endotoxin contamination levels are hidden behind the trade secrets act by the label of adjuvant.
Out of curiosity have you looked into that at all mate?