MANUFACTURER’S CONVENIENCE FOR RESIDUAL DNA
The FDA Briefing Document. Vaccines and Related Biological Products Advisory Committee Meeting September 19, 2012: Cell Lines Derived from Human Tumors for Vaccine Manufacture.
FDA DOCUMENT
This FDA document teaches us the risks associated with the residual DNA. Let's take a look at some interesting statements about the regulation value of 10 ng for the residual DNA (see P.25).
First, vaccine manufacturers could not always meet this level of residual cell-substrate DNA for some viral vaccines, such as with certain enveloped viruses.
Second, more information was available as to the oncogenic events in human cancers, where it has been established that multiple events, both genetic and epigenetic, are required (31, 32, 89, 98, 99).
And third, for continuous non-tumorigenic cell lines such as Vero, the major cell substrate that was being considered at the time, the presence of activated dominant oncogenes in these cells was unlikely.
When the first to third reasons are applied to the DNA wrapped in the LNP (LNP-DNA), the second and third reasons are not appropriate for the LNP-DNA because they did not take the LNP into account. The LNP had not yet been invented in 1997.
That is, for the LNP-DNA, the regulation value of 10 ng is based solely on the first reason, "manufacturer's convenience."
In my field, it is commonly believed that Arbutus Biopharma Corp holds the dominant patent US8058069B2 for the LNP. This patent was filed on April 15, 2009, and granted on November 15, 2011.
CITATION 10
Even looking at the citation 10, no specific information was found. Needless to say, this paper does not provide justifiable reason not to take the LNP into account.
The outcome of the 1997 WHO meeting was that the amount of residual cell-substrate DNA allowed per dose in a vaccine produced in a continuous cell line and one administered by the parenteral route was raised from 100 pg to 10 ng (10).
WHO DOCUMENT
The WHO document published in 1998, the year after 1997, contained more detailed information (see page 25), but I could not find anything equivalent to the FDA document.
However, this WHO document has an interesting information.
The new upper limit of 10ng of residual DNA per dose does not apply to products derived from microbial, diploid or primary-cell-culture systems. The 1986 WHO Study Group stated that the risks for con-tinuous-cell-line DNA should be considered negligible for preparations given orally; for such products, the principal requirement is the elimination of potentially contaminating viruses and toxic proteins.
The upper limit of 10ng of residual continuous-cell-line DNA per dose therefore does not apply to a product given orally. Acceptable limits should be set in consultation with the national control author-ity.
The critics of the DNA contamination issue sometimes contrast the DNA that contaminates vaccines with the food DNA. In this point, the 10 ng residual DNA contamination limit does not apply to the oral vaccines (see WHO document, p. 25 to 27). This is because the risk is lower compared to subcutaneous or intramuscular injections. In the case of the oral products , the DNA is broken down during the digestion process.
Such structure of regulation also highlights the inconsistency of discussing food DNA and DNA contaminating vaccines in the same category.
LNP Dominant Patent US8058069B2
Current Assignee: Arbutus Biopharma
Filing date:April 15, 2009
Registration date: November 15, 2011
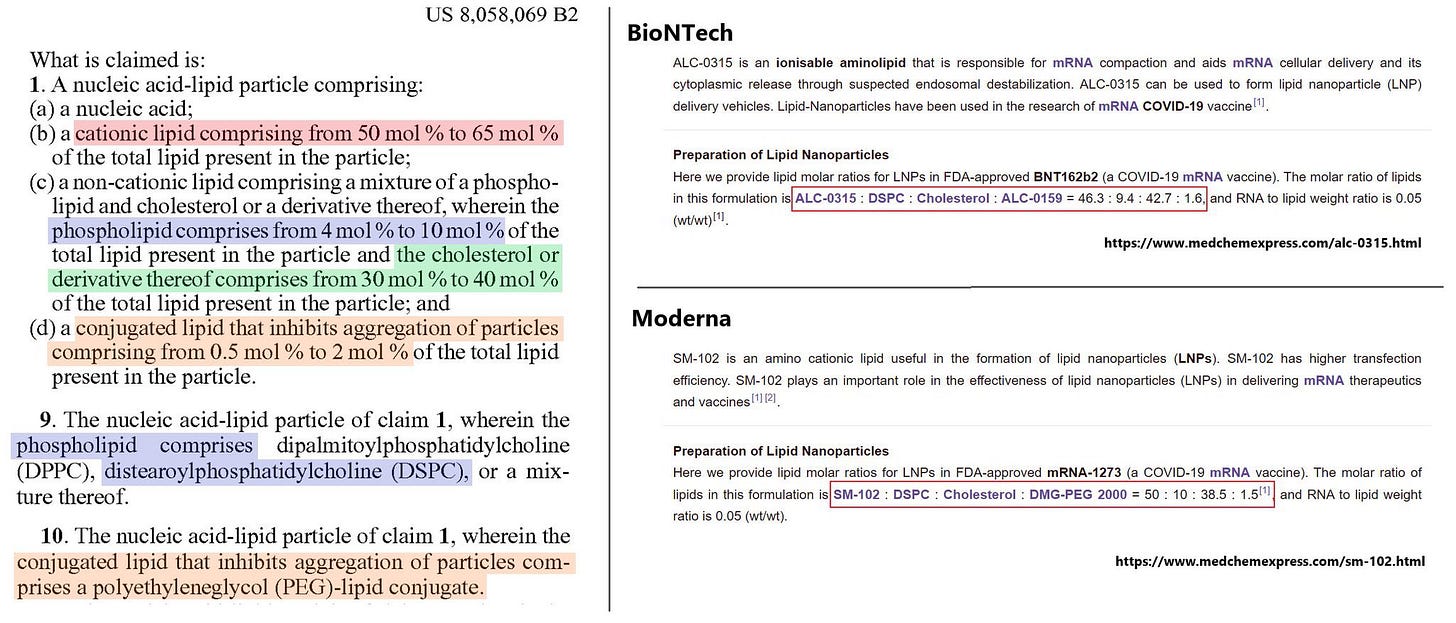
US8058069B2, often referred to as the “’069 patent,” is considered to be a dominant patent in LNP field because it outlines foundational methods for delivering nucleic acids using LNP. This patent is critical as LNP are a key enabling technology for mRNA vaccines, including those developed by BioNTech and Moderna.
The litigation between Arbutus and companies like BioNTech and Moderna centers on whether their use of LNP technology for mRNA vaccines infringes on this patent.
The correspondence between the Patented LNP, BioNTech LNP, and Moderna LNP is shown in the following table.
BioNTech LNP infringe the patent except for the cholesterol, suggesting that it seems to be designed to avoid the patent. On the other hand, in the case of Moderna, the SM-102 is actually a lipid whose cationization depends on pH, raising the issue of whether it falls under the limitaion of the “cationic lipid” in the patent. In practice, since the SM-102 becomes cationized within cells, it is highly likely to fall within the scope of the “cationic lipids.” Anyways, Moderna LNP also seems to be designed to avoid the patent.
As patent protection extends not only to exact matches but also to substantially equivalent variations, their dispute is taking on the appearance of a protracted legal battle.
The above FDA document contains multiple sources of information and will be of public interest. Please let me know if you have any information related to those, especially information that provides justification for not considering LNP in the residual DNA regulation values.










Great information, thanks. Have just added here:
https://geoffpain.substack.com/p/bacterial-dna-a-major-worry-in-jabs
Thanks!