ABRYSVO
Pfizer RSV vaccine for pregnant women
In Japan, the death of an Abrysvo recipient was reported on September 12, 2024. The victim was a 30-year-old woman who received the Abrysvo on August 20, 2024. The death occurred less than one month after the vaccination date, and the specific cause of death has not been disclosed yet.
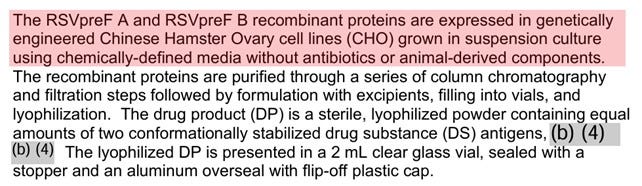
In the clinical trials of the Abrysvo, a lyophile containing no active ingredients was used as a placebo, instead of the pure saline, as usual. The possibility that the aluminum adjuvant Al(OH)3 was used in the placebo is not completely ruled out due to clever wording. Note that there is no aluminum adjuvant in the approved Abrysvo.
The placebo group also experienced a certain amount of side effects (i.e. side effects of the drug other than the active ingredient). Some are quoted here.
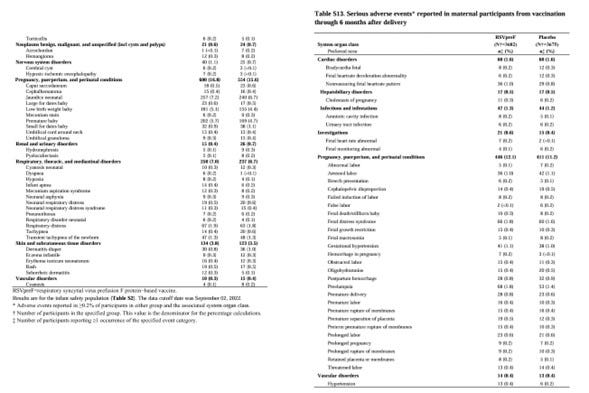
The Abrysvo has been approved for use to the pregnant women in the late stages of pregnancy, from 24 to 36 weeks (recommended from 28 weeks), due to the risk of premature birth. However, the cause of the premature birth has not been identified yet. Even if the premature birth occurs after vaccination due to the Abrysvo, it is unclear whether it is due to the Abrysvo or not.
The gestational hypertension syndrome is classified into early type (up to 32-34 weeks) and late type (after 32-34 weeks). The proportion of early type is said to be about 10-25%, and the proportion of late type is said to be about 75-90%.
Here's another trick that the FDA and Pfizer did.
They matched the risk of gestational hypertension syndrome caused by the Abrysvo to the high-risk period for the late gestational hypertension syndrome in the pregnant women. This makes it difficult to determine whether the gestational hypertension syndrome is caused by the Abrysvo or purely by the pregnancy.
In Japan, the number of older women giving birth is also increasing, so it is expected that the cause will be replaced by this.
To confirm the effectiveness of the Abrysvo, it is necessary to actively promote the vaccination of the Abrysvo to the pregnant women with urinary protein and/or high blood pressure. After vaccination, an increase in urinary protein and/or high blood pressure will be confirmed, and the resulting inhibition of fetal growth will also be confirmed. It seems necessary to closely monitor the fetal growth after vaccination.
At the time of birth, the labor will likely be induced before the due date, with a Caesarean section also in mind. If the labor is not induced as desired, the blood pressure of the pregnant women may rise abnormally, putting both the mother and the fetus at risk of dying, and an emergency Caesarean section may be required.
It is expected that the rate of Caesarean sections will increase in the patients receiving the Abrysvo in the future. It is also expected that the number of complications of gestational hypertension syndrome (eclampsia, HELLP syndrome, etc.) will increase after childbirth.
Why did the FDA and Pfizer allow the Abrysvo to the pregnant women who show symptoms of the gestational hypertension syndrome, despite being aware of the risk of this?
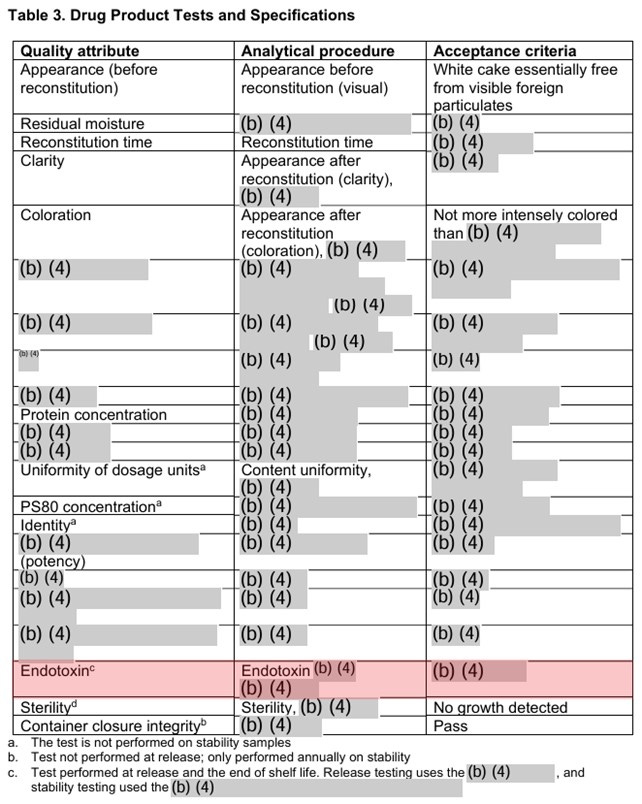
In the first place, the inactivated vaccines are inevitably contaminated by residual DNA and endotoxin derived from animal cell (Chinese Hamster Ovary cell in the case of Abrysvo) as well as formalin. Is it no problem to introduce toxic substances such as endotoxin (lipopolysaccharide) to the pregnant women with weakened immune systems?
For further discussion on the endotoxin in the Abrysvo, please see the article from Geoff Pain san.
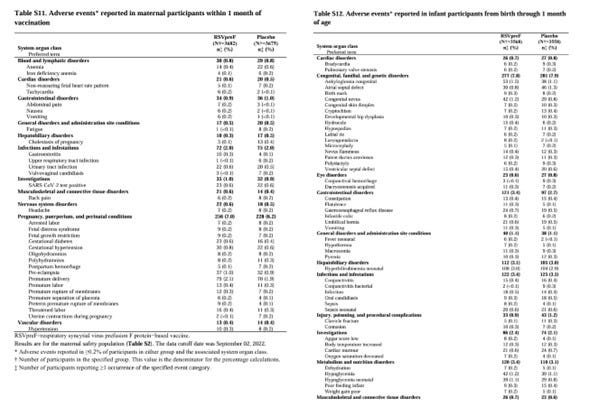
The results showed that there were more cases of the gestational hypertension syndrome in the drug group compared to the placebo group, but the placebo is the drug without the active ingredient, probably the RSV as an antigen.
Comparing the placebo group and the drug group for the premature birth and the gestational hypertension syndrome does not deny the possibility that those side effects were induced by some ingredient contained in both groups.
With the placebo that does not use the pure saline, it is possible to confirm the effectiveness of the antigen (the effect of increasing antibody titers), but safety will not be guaranteed. Therefore, there is a concern that the adverse events will increase significantly in the real world.
Reading agenda item 4 of the "Minutes of the Pharmaceutical Affairs and Food Sanitation Council, Second Committee on Drugs, November 27, 2023," the comparison of the drug group and the placebo group was made without anyone checking the definition of the placebo for the Abrysvo or questioning it.
Since it is in Japanese language, I will quote just one sentence in English translation below.
The incidence rates were similar between the drug group and the placebo group, at 5.6% in the drug group and 4.7% in the placebo group, and in the Japanese subgroup, at 3% in the drug group and 5.6% in the placebo group, so there was no concern about the premature birth due to the Abrysvo vaccination.
I wonder if all the participants here are mistaken in thinking that the placebo is pure saline.




Thank you for your efforts, PatentSun.
RSVirus (negative single-stranded RNA virus, about 150nmφ, spherical to ellipsoidal, with envelope, many protrusions for cell fusion)
https://en.wikipedia.org/wiki/Respiratory_syncytial_virus
According to observations by medical institutions in Tokyo, the peak infection rate at hospitals is less than 0.02% of the total population, more in spring, summer, and fall, and lower in winter.
Problems with the clinical trial certificate for Pfizer's Abrysvo (for pregnant women)
1. Clinical trials are conducted by Pfizer personnel = Interest of Conflict is significant.
2. Infection is determined by PCR method = similar to the determination of Cov19, there is a large margin of error, and antigen tests are also uncertain.
3. As PatentSun says, unless Placebo = Saline, it is fraud.
There is a similar example.
Sequirus Flucelvax® Quad (for influenza A, B) ARR = 17.5%, RRR = 51.9%
However, Placebo is administered with meningococcal ACWY instead of saline.
Meningococcus can cause pneumonia when it is secondarily infected in influenza patients,
but in short, this is just a fraudulent clinical trial and Flucelvax is more effective against influenza than ACWY-vax.
The FDA, which was bribed to reserve a new job, has approved it with this.
The two people who approved BNT162b2 etc. at the FDA in 2023 moved to Pfizer.
In 2016, 15 of 26 FDA members moved to pharmaceutical-related companies.
In 2018, 11 of 16 FDA members moved to pharmaceutical-related companies.
I think the same is true for Abrysvo approval.
A man named Fujiwara, a Biotech faction member who was dispatched from the FDA, has infiltrated the PMDA, the drug approval organization in Japan, and Replicon(Sarscov2) has been approved.
④ Of the Absolute (ARR) and Relative (RRR) efficacy, the RRR display is misleading to doctors and laypeople.
If we roughly assume 3700*2 groups (vaccinated group and placebo group), the efficacy based on ARR is
(62-19)/3700=1.1%: Severe
(117-57)/3700=1.6%: LowerTract
For reference, here are the ARR figures for other companies' RSV-vaccines that I calculated.
GSK's Arexvy (RSV) ARR = 0.26%, RRR = 82.5%
Moderna's mRNA-1345 (RSV) ARR = 0.53%, RRR = 68.4%
Although it is more pharmaceutical-oriented, the MSD manual states that
"There is currently no vaccine that can prevent infection with RS virus or hMPV.
If the patient has difficulty breathing, they will be taken to the hospital and treated with oxygen inhalation or infusion depending on their condition."
However, it is said that the infection is often serious in infants under 1 year old and elderly people with respiratory diseases.
Many doctors say that "it can be adequately treated with a nebulizer (β2 agonist, Vavirin, ...)."
⑤ Important qualitative rules regarding substances injected into the bloodstream:
There is an old saying in Japan that dates back to when no one knows. "Be careful of twice sting by bee. (there is a high chance of death if you are stung a second time by bee, even if you don't die, you'll feel sick.)"
People in the past didn't have science, but they pointed out the main points from experience.
The person who medically demonstrated this was Charles Robert Richet (August 25, 1850 - December 4, 1935), who won a Nobel Prize.
https://www.nobelprize.org/prizes/medicine/1913/richet/lecture/
An easy-to-understand explanation of this.
https://sashalatypova.substack.com/p/the-second-shot-or-what-do-vaccinators?publication_id=870364
I think the vaccine industry has been abusing Richet's law for over 100 years.
You might like
https://geoffpain.substack.com/p/endotoxin-on-pfizer-abrysvo-respiratory